US bins 60m contaminated Johnson and Johnson covid jabs
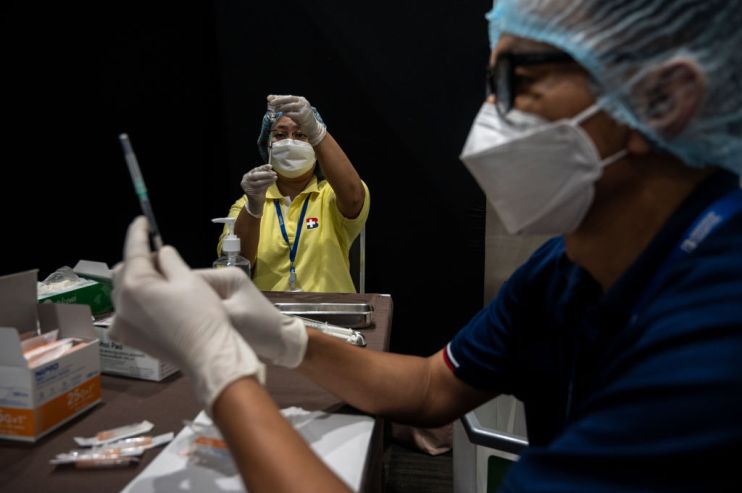
Regulators in the US have ruled that 60 million doses of the Johnson & Johnson coronavirus vaccine will need to be discarded because of suspected contamination, according to reports.
The doses were produced in a factory in Baltimore operated by Emergent BioSolutions, where federal regulators have been conducting a review over the course of several weeks.
The Food and Drug Administration (FDA) has yet to decide if Emergent can reopen the premises, which have been closed for around two months as a result of the regulatory concerns.
The FDA plans to allow the distribution of 10 million doses across the United States or to other countries, but would issue a warning they cannot guarantee that Emergent followed good manufacturing practices.
The fate of over 170 million doses of vaccine have been left uncertain after they were put on hold when a contamination was discovered.
Emergent workers accidentally contaminated a batch of Johnson & Johnson’s vaccine with a key ingredient used to produce AstraZeneca’s leaving the FDA unsure of what to do with 100 million Johnson & Johnson vaccines, and 70 million AstraZeneca doses.
Emergent production was paused and its responsibility to produce the AstraZeneca vaccine stripped. The FDA ordered Johnson & Johnson to assert direct control over the manufacturing of its vaccine there.
The Biden administration had planned on sharing doses of both Johnson & Johnson and AstraZeneca vaccines as part of its strategy to distribute vaccines to other countries, but it was forced to delay this while the FDA completed a review of the Baltimore facility.