Shire jumps to record as ADHD drug is used for new disorder
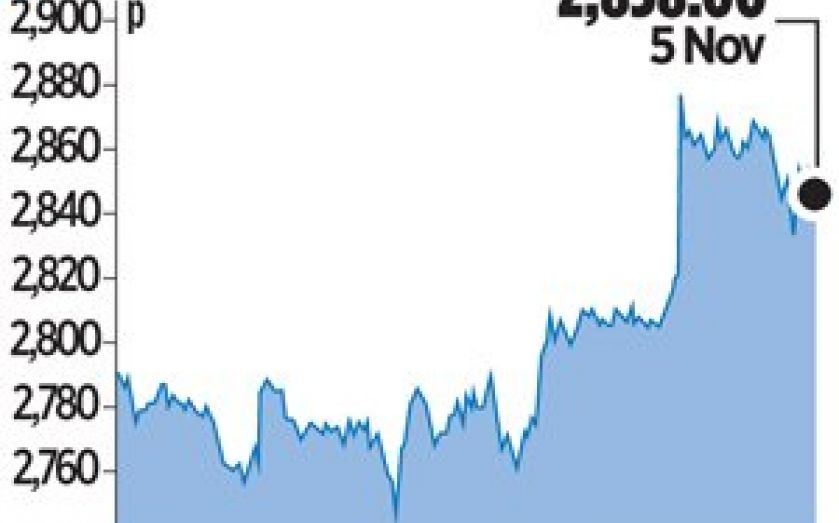
PHARMACEUTICAL group Shire yesterday said Vyvanse, its amphetamine-based drug prescribed to US students to control ADHD, had also been successful in treating the newly-recognised binge eating disorder (BED) in a trial.
Shares in the London-listed firm rose to an all-time high after it said the drug was superior to a placebo in reducing the number of binge-eating days per week in two randomised late-stage trials.
It said it would submit an application to the US drugs regulator, the FDA, for the approval of the drug for BED after the results of the tests, which were completed earlier than expected.
Shire’s chief executive Flemming Ornskov, who joined the company earlier this year, said he was extremely pleased with the results.
“BED is a condition for which there is no currently approved pharmacologic treatment and yet there is significant unmet patient need, as was demonstrated with the faster than expected enrolment of participants in our clinical trial programme,” he said.
Shire said it expected to file for FDA approval by the third quarter of 2014.