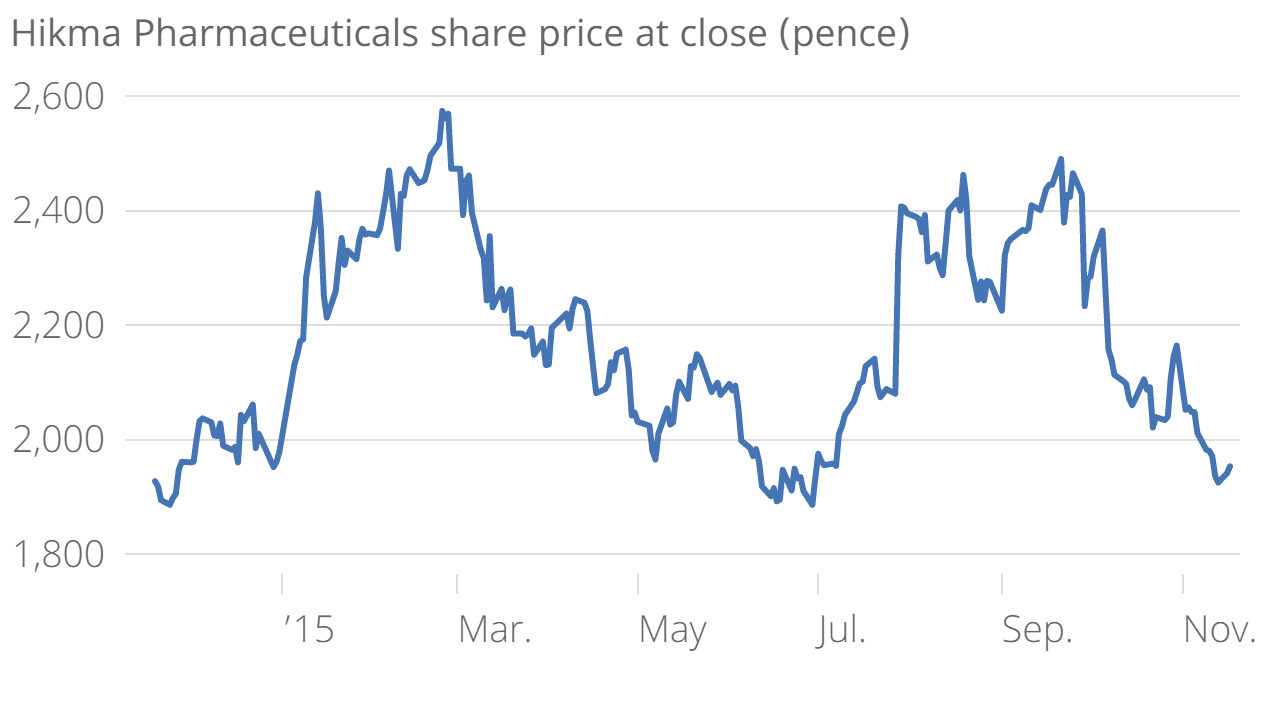
Hikma Pharmaceuticals share price rises on improved market prospects in US
London-listed Hikma Pharmaceuticals received a boost this morning, after the FDA said it no longer had an issue with the company's injectibles manufacturing plant in Portugal.
The US regulatory body said it was “closing” a warning letter it sent to the firm in October last year, in which it threatened to block drugs manufactured at the plant from entering the US market unless the facilities were improved.
In particular, the FDA took issue with the factory's environmental standards and the lack of adequate production controls. It said this could compromise the strength and quality of the drugs produced.
At the time, it was a major knock to the Jordanian pharmaceutical company, but today's news marks an improvement in trade prospects in the US. Hikma said the “corrective actions that were taken in response to the warning letter were fully reviewed and accepted by the US FDA”.
Sarah Darwazah, chairman and chief executive of Hikma, said: “I am very pleased that we have brought our Portuguese facility back into compliance with the US FDA. We have worked very hard to meet the FDA’s requirements and remain committed to maintaining the highest standards of quality and compliance across all our facilities.”
We believe that the resolution of the warning letter will enable us to accelerate the introduction of new products to the market, ensuring we continue to broaden the range of critical medicines we supply to patients in the US.
Hikma was the second highest riser on the LSE this morning following the news, with shares currently up 2.7 per cent at 2,005 pence.