MPs call out ‘complete absence’ of invasive beauty treatment regulation
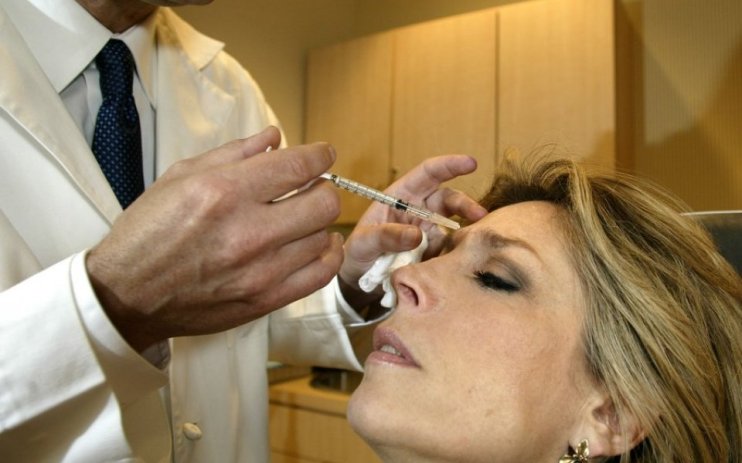
MPs have urged the government to strengthen regulation on non-surgical beauty treatments, like fillers and injections, citing a “complete absence” of a legal framework.
After a year-long review, the All-Party Parliamentary Group (APPG) on Beauty, Aesthetics and Wellbeing have claimed the government has left the young industry to regulate itself.
The APPG advised mandatory training for all practitioners, in a report published today, as beauty procedures – which often do not result in complications – can put patients at risk if not carried out properly.
Although botox-style injections and fillers have been popular for some time, carried by social media celebrities like Kylie Jenner and Kim Kardashian, an increasing number of new and obscure treatments are emerging.
Invasive treatments like thread lifts have soared in popularity, but unlike botox, are not yet ruled out for those under 18.
“It’s like the Wild West,” inquiry co-chairwoman and Labour MP Carolyn Harris explained.
“We have people who are selling training courses which are not worth the paper they are written on. We have practitioners who are destroying the industry’s reputation by practising completely unqualified and we have victims who are scarred for life.”
Patient safety minister Nadine Dorries is set to review the report.
Recommendations
The review has made 17 recommendations that it wants the government to implement, which the Department for Health and Social Care will review.
Beyond mandatory training, the APPG has urged for a national licensing system that ensures premises meet certain hygiene requirements – which helps to combat ‘pop-up’ shop style practices.
The report also advised mandatory psychological screenings ahead of treatments, so patients understand that there are no ‘quick fixes’ to self-esteem issues.
The APPG recommended that dermal fillers be classified as a ‘Prescription Only Medicine’ – as well as extending the under-18 bad to emerging treatments.
“Advertising restrictions should be placed on dermal fillers and PDO cogs and threads in
the same way as those imposed on botox as a Prescription Only Medicine,” the report added.
It urged that online marketing and advertising of such treatments should be monitored.